Localized SPR for Pb(II) ion sensing utilizing chitosan/reduced graphene oxide nanocomposite
DOI:
https://doi.org/10.53655/joe.z3238qKeywords:
Surface plasmon resonance, chitosan, graphene oxide, nanocomposite, heavy metalAbstract
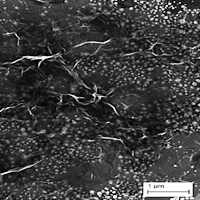
In this work, a simple and highly sensitive localized surface plasmon resonance (LSPR) technique had been developed for detection of Pb(II) utilizing chitosan/reduced graphene oxide (CS/rGO) nanocomposite as the sensing material. Gold nanoparticles (AuNP) had been incorporated with the nanocomposite material to induce strong LSPR responses. CS/rGO nanocomposite was coated on top of AuNP and had been utilized as active layer for Pb(II) ion sensing. The morphology and physical properties of gold nanoparticle-chitosan/reduced graphene oxide (AuNP-CS/rGO) nanocomposite was studied using field-emission scanning electron microscopy (FESEM), atomic force microscopy (AFM) and x-ray diffraction (XRD) analysis. FESEM exhibits a rough and wrinkle surface of AuNP-CS/rGO after the addition of rGO. AFM shows a higher surface roughness for AuNP-CS/rGO due to the presence of oxygen atoms in rGO where these oxygen atoms provide more sites for adsorption of Pb(II), hence enhance the sensitivity of the sensor. A good sensitivity of the LSPR sensor for detection of Pb(II) utilizing AuNP-CS/rGO was obtained which is 1.544 ppm-1 and the linearity of the sensor are 0.99 and 0.97. In stability study, AuNP-CS/rGO exhibits a good repeatability with relative standard deviation of 2% for 0.01 ppm.
References
S. Abdullah, N.H. Azeman, N.N. Mobarak, M.S.D. Zan, A.A. A. Bakar, Sensitivity enhancement of localized SPR sensor towards Pb(II) ion detection using natural bio-polymer based carrageenan, Optik (Stuttg). 168 (2018). https://doi.org/10.1016/j.ijleo.2018.05.016.
N.F. Lokman, N.H. Azeman, F. Suja, N. Arsad, A.A.A. Bakar, Sensitivity enhancement of Pb(II) ion detection in rivers using SPR-based Ag metallic layer coated with chitosan–graphene oxide nanocomposite, Sensors (Switzerland). (2019). https://doi.org/10.3390/s19235159.
N.H. Kamaruddin, A.A.A. Bakar, M.H. Yaacob, M.A. Mahdi, M.S.D. Zan, S. Shaari, Enhancement of chitosan-graphene oxide SPR sensor with a multi-metallic layers of Au-Ag-Au nanostructure for lead(II) ion detection, Appl. Surf. Sci. 361 (2016). https://doi.org/10.1016/j.apsusc.2015.11.099.
J.H. Park, J.Y. Byun, S.Y. Yim, M.G. Kim, A Localized Surface Plasmon Resonance (LSPR)-based, simple, receptor-free and regeneratable Hg2+ detection system, J. Hazard. Mater. 307 (2016). https://doi.org/10.1016/j.jhazmat.2015.12.040.
J. Bi, M. Fang, J. Wang, S. Xia, Y. Zhang, J. Zhang, G. Vegesna, S. Zhang, M. Tanasova, F.T. Luo, H. Liu, Near-infrared fluorescent probe for sensitive detection of Pb(II) ions in living cells, Inorganica Chim. Acta. 468 (2017). https://doi.org/10.1016/j.ica.2017.06.044.
H. Ebrahimzadeh, M. Behbahani, A novel lead imprinted polymer as the selective solid phase for extraction and trace detection of lead ions by flame atomic absorption spectrophotometry: Synthesis, characterization and analytical application, Arab. J. Chem. 10 (2017). https://doi.org/10.1016/j.arabjc.2013.09.017.
G. Xing, M.R. Sardar, B. Lin, J.M. Lin, Analysis of trace metals in water samples using NOBIAS chelate resins by HPLC and ICP-MS, Talanta. 204 (2019). https://doi.org/10.1016/j.talanta.2019.05.041.
T. Feng, J. Xu, C. Yu, K. Cheng, Y. Wu, Y. Wang, F. Li, Graphene oxide wrapped melamine sponge as an efficient and recoverable adsorbent for Pb(II) removal from fly ash leachate, J. Hazard. Mater. 367 (2019). https://doi.org/10.1016/j.jhazmat.2018.12.053.
A.R. Sadrolhosseini, M. Naseri, S.A. Rashid, Polypyrrole-chitosan/nickel-ferrite nanoparticle composite layer for detecting heavy metal ions using surface plasmon resonance technique, Opt. Laser Technol. 93 (2017). https://doi.org/10.1016/j.optlastec.2017.03.008.
G. Qiu, S.P. Ng, X. Liang, N. Ding, X. Chen, C.M.L. Wu, Label-Free LSPR Detection of Trace Lead(II) Ions in Drinking Water by Synthetic Poly(mPD-co-ASA) Nanoparticles on Gold Nanoislands, Anal. Chem. 89 (2017). https://doi.org/10.1021/acs.analchem.6b04536.
B. Feng, R. Zhu, S. Xu, Y. Chen, J. Di, A sensitive LSPR sensor based on glutathione-functionalized gold nanoparticles on a substrate for the detection of Pb2+ ions, RSC Adv. 8 (2018). https://doi.org/10.1039/c7ra13127e.
G.Y. Qiu, A.H.L. Law, S.P. Ng, C.M.L. Wu, Label-free Detection of Lead(II) Ion Using Differential Phase Modulated Localized Surface Plasmon Resonance Sensors, in: Procedia Eng., 2016. https://doi.org/10.1016/j.proeng.2016.11.516.
K.A. Willets, R.P. Van Duyne, Localized surface plasmon resonance spectroscopy and sensing, Annu. Rev. Phys. Chem. 58 (2007). https://doi.org/10.1146/annurev.physchem.58.032806.104607.
L. Singh, R. Singh, B. Zhang, S. Cheng, B. Kumar Kaushik, S. Kumar, LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber, Opt. Fiber Technol. 53 (2019). https://doi.org/10.1016/j.yofte.2019.102043.
M.E. Martínez-Hernández, J. Goicoechea, F.J. Arregui, Hg2+ optical fiber sensor based on LSPR generated by gold nanoparticles embedded in LBL nano-assembled coatings, Sensors (Switzerland). 19 (2019). https://doi.org/10.3390/s19224906.
T.J. Lin, M.F. Chung, Using monoclonal antibody to determine lead ions with a localized surface plasmon resonance fiber-optic biosensor, Sensors. 8 (2008). https://doi.org/10.3390/s8010582.
M. Guo, W.C. Law, X. Liu, H. Cai, L. Liu, M.T. Swihart, X. Zhang, P.N. Prasad, Plasmonic Semiconductor Nanocrystals as Chemical Sensors: Pb2+ Quantitation via Aggregation-Induced Plasmon Resonance Shift, Plasmonics. 9 (2014). https://doi.org/10.1007/s11468-014-9694-3.
N.F. Lokman, A.A.A. Bakar, S.A. Talib, F. Suja, SPR response of CS-GO nanostructured thin films for selective detection of Pb(II) ions in the Saigon River, Vietnam, Desalin. Water Treat. 93 (2017). https://doi.org/10.5004/dwt.2017.21407.
N.H. Azeman, N. Arsad, A.A.A. Bakar, Polysaccharides as the sensing material for metal ion detection-based optical sensor applications, Sensors (Switzerland). 20 (2020). https://doi.org/10.3390/s20143924.
H. Patel, D. Rawtani, Y.K. Agrawal, A newly emerging trend of chitosan-based sensing platform for the organophosphate pesticide detection using Acetylcholinesterase- a review, Trends Food Sci. Technol. 85 (2019). https://doi.org/10.1016/j.tifs.2019.01.007.
G.S. Gadgey, K.K., Sharma, Investigation of mechanical properties of chitosan based films: a review, Int. J. Adv. Res. Eng. Technol. 8 (2017).
Y. Jiang, J. Wu, Recent development in chitosan nanocomposites for surface-based biosensor applications, Electrophoresis. 40 (2019). https://doi.org/10.1002/elps.201900066.
R. Justin, B. Chen, Strong and conductive chitosan-reduced graphene oxide nanocomposites for transdermal drug delivery, J. Mater. Chem. B. 2 (2014). https://doi.org/10.1039/c4tb00390j.
M.N. Muralidharan, K.P. Shinu, A. Seema, Optically triggered actuation in chitosan/reduced graphene oxide nanocomposites, Carbohydr. Polym. 144 (2016). https://doi.org/10.1016/j.carbpol.2016.02.047.
Y. Zhu, D. Pan, X. Hu, H. Han, M. Lin, C. Wang, An electrochemical sensor based on reduced graphene oxide/gold nanoparticles modified electrode for determination of iron in coastal waters, Sensors Actuators, B Chem. 243 (2017). https://doi.org/10.1016/j.snb.2016.11.108.
N.F. Lokman, A.A.A. Bakar, F. Suja, H. Abdullah, W.B.W.A. Rahman, N.M. Huang, M.H. Yaacob, Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb (II) ions, Sensors Actuators, B Chem. 195 (2014). https://doi.org/10.1016/j.snb.2014.01.074.
Z. Lu, S. Yang, Q. Yang, S. Luo, C. Liu, Y. Tang, A glassy carbon electrode modified with graphene, gold nanoparticles and chitosan for ultrasensitive determination of lead(II), Microchim. Acta. 180 (2013). https://doi.org/10.1007/s00604-013-0959-x.
J.Z. Zhang, C. Noguez, Plasmonic optical properties and applications of metal nanostructures, Plasmonics. 3 (2008). https://doi.org/10.1007/s11468-008-9066-y.
D.T. Nurrohman, N.F. Chiu, A review of graphene-based surface plasmon resonance and surface-enhanced raman scattering biosensors: Current status and future prospects, Nanomaterials. 11 (2021). https://doi.org/10.3390/nano11010216.
Y. Zuo, J. Xu, F. Jiang, X. Duan, L. Lu, H. Xing, T. Yang, Y. Zhang, G. Ye, Y. Yu, Voltammetric sensing of Pb(II) using a glassy carbon electrode modified with composites consisting of Co3O4 nanoparticles, reduced graphene oxide and chitosan, J. Electroanal. Chem. 801 (2017). https://doi.org/10.1016/j.jelechem.2017.07.046.
Z. Guo, D. di Li, X. ke Luo, Y. hui Li, Q.N. Zhao, M. meng Li, Y. ting Zhao, T. shuai Sun, C. Ma, Simultaneous determination of trace Cd(II), Pb(II) and Cu(II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-L-lysine nanocomposite modified glassy carbon electrode, J. Colloid Interface Sci. 490 (2017). https://doi.org/10.1016/j.jcis.2016.11.006.
A.A. Umar, I. Iwantono, A. Abdullah, M.M. Salleh, M. Oyama, Gold nanonetwork film on the ito surface exhibiting one-dimensional optical properties, Nanoscale Res. Lett. 7 (2012). https://doi.org/10.1186/1556-276X-7-252.
A.A. Umar, M. Oyama, Attachment of gold nanoparticles onto indium tin oxide surfaces controlled by adding citrate ions in a seed-mediated growth method, Appl. Surf. Sci. 253 (2006). https://doi.org/10.1016/j.apsusc.2006.06.038.
J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang, S. Guo, Reduction of graphene oxide vial-ascorbic acid, Chem. Commun. 46 (2010). https://doi.org/10.1039/b917705a.
L. Polavarapu, J. Pérez-Juste, Q.H. Xu, L.M. Liz-Marzán, Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles, J. Mater. Chem. C. 2 (2014). https://doi.org/10.1039/c4tc01142b.
J. Zhang, M. Oyama, Gold nanoparticle arrays directly grown on nanostructured indium tin oxide electrodes: Characterization and electroanalytical application, Anal. Chim. Acta. 540 (2005). https://doi.org/10.1016/j.aca.2005.03.054.
Y.W. Lin, C.C. Huang, H.T. Chang, Gold nanoparticle probes for the detection of mercury, lead and copper ions, Analyst. 136 (2011). https://doi.org/10.1039/c0an00652a.
A. Sugunan, C. Thanachayanont, J. Dutta, J.G. Hilborn, Heavy-metal ion sensors using chitosan-capped gold nanoparticles, in: Sci. Technol. Adv. Mater., 2005. https://doi.org/10.1016/j.stam.2005.03.007.
Y. Shen, J. Zhou, T. Liu, Y. Tao, R. Jiang, M. Liu, G. Xiao, J. Zhu, Z.K. Zhou, X. Wang, C. Jin, J. Wang, Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit, Nat. Commun. 4 (2013). https://doi.org/10.1038/ncomms3381.
M.M. Beppu, R.S. Vieira, C.G. Aimoli, C.C. Santana, Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption, J. Memb. Sci. 301 (2007). https://doi.org/10.1016/j.memsci.2007.06.015.
W. Wang, Y. He, N. Zhang, W. Wang, S. He, Q. Gong, H. Qiu, Preparation of reduced graphene oxide/chitosan composite films with reinforced mechanical strength, in: Adv. Mater. Res., 2012. https://doi.org/10.4028/www.scientific.net/AMR.430-432.247.
J. Li, H. Feng, J. Li, Y. Feng, Y. Zhang, J. Jiang, D. Qian, Fabrication of gold nanoparticles-decorated reduced graphene oxide as a high performance electrochemical sensing platform for the detection of toxicant Sudan i, Electrochim. Acta. 167 (2015). https://doi.org/10.1016/j.electacta.2015.03.201.
D. Konios, M.M. Stylianakis, E. Stratakis, E. Kymakis, Dispersion behaviour of graphene oxide and reduced graphene oxide, J. Colloid Interface Sci. 430 (2014). https://doi.org/10.1016/j.jcis.2014.05.033.
Y. Fang, D. Zhang, Y. Guo, Y. Guo, Q. Chen, Simple one-pot preparation of chitosan-reduced graphene oxide-Au nanoparticles hybrids for glucose sensing, Sensors Actuators, B Chem. 221 (2015). https://doi.org/10.1016/j.snb.2015.06.098.
Y. Pan, T. Wu, H. Bao, L. Li, Green fabrication of chitosan films reinforced with parallel aligned graphene oxide, Carbohydr. Polym. 83 (2011). https://doi.org/10.1016/j.carbpol.2010.10.054.
P. Sharma, G. Darabdhara, T.M. Reddy, A. Borah, P. Bezboruah, P. Gogoi, N. Hussain, P. Sengupta, M.R. Das, Synthesis, characterization and catalytic application of Au NPs-reduced graphene oxide composites material: An eco-friendly approach, Catal. Commun. 40 (2013). https://doi.org/10.1016/j.catcom.2013.06.021.
J. Gong, X. Hu, K.W. Wong, Z. Zheng, L. Yang, W.M. Lau, R. Du, Chitosan nanostructures with controllable morphology produced by a nonaqueous electrochemical approach, Adv. Mater. 20 (2008). https://doi.org/10.1002/adma.200701840.
J. Rodríguez-Fernández, A.M. Funston, J. Pérez-Juste, R.A. Álvarez-Puebla, L.M. Liz-Marzán, P. Mulvaney, The effect of surface roughness on the plasmonic response of individual sub-micron gold spheres, Phys. Chem. Chem. Phys. 11 (2009). https://doi.org/10.1039/b905200n.
B. Zahed, H. Hosseini-Monfared, A comparative study of silver-graphene oxide nanocomposites as a recyclable catalyst for the aerobic oxidation of benzyl alcohol: Support effect, Appl. Surf. Sci. 328 (2015). https://doi.org/10.1016/j.apsusc.2014.12.078.
U. Guler, R. Turan, Effect of particle properties and light polarization on the plasmonic resonances in metallic nanoparticles, Opt. Express. 18 (2010). https://doi.org/10.1364/oe.18.017322.
K.R. Catchpole, A. Polman, Plasmonic solar cells, Opt. Express. 16 (2008). https://doi.org/10.1364/oe.16.021793.
J. Cao, E.K. Galbraith, T. Sun, K.T.V. Grattan, Effective surface modification of gold nanorods for localized surface plasmon resonance-based biosensors, Sensors Actuators, B Chem. 169 (2012). https://doi.org/10.1016/j.snb.2012.05.019.
Y.-T. Long, C. Jing, Localized surface plasmon resonance based nanobiosensors., 2014.
K. Ariga, M. Aono, Nanoarchitectonics, Jpn. J. Appl. Phys. 55 (2016). https://doi.org/10.7567/JJAP.55.1102A6.
C.L. Tan, S.J. Jang, Y.T. Lee, Localized surface plasmon resonance with broadband ultralow reflectivity from metal nanoparticles on glass and silicon subwavelength structures, Opt. Express. 20 (2012). https://doi.org/10.1364/oe.20.017448.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Suraya Abdullah, Nur Hidayah Azeman, Nur Hasiba Kamaruddin, Nadhratun Naiim Mobarak, Ahmad Ashrif A Bakar

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.



